Exploring Antioxidant and Anti-diabetic Activities, and Chemical Contents of Extracts from Thai Traditional Medicine (Pra-Sa-Ka-Phrao Remedies) and Its Plant Ingredients
Keywords:
Pra-Sa-Ka-Phrao remedy, Antioxidant activity, Anti-diabetic activity, Chemical content, Thai traditional medicineAbstract
Introduction: Pra-Sa-Ka-Phrao complete (PSKPC) remedy is a Thai traditional medicine published in the Thailand National List of Essential Medicines (NLEM). In this research, we have developed a modified version of the remedy, named as Pra-Sa-Ka-Phrao incomplete (PSKPIC), following the FDA Thailand’s guidelines for using it as a food supplement. Notably, there is a lack of studies concerning biological activities and chemical constituents of both remedies.
Objectives: This study aimed to investigate and compare the antioxidant and anti-diabetic activities, and chemical contents derived from both remedies and its plant ingredient extracts.
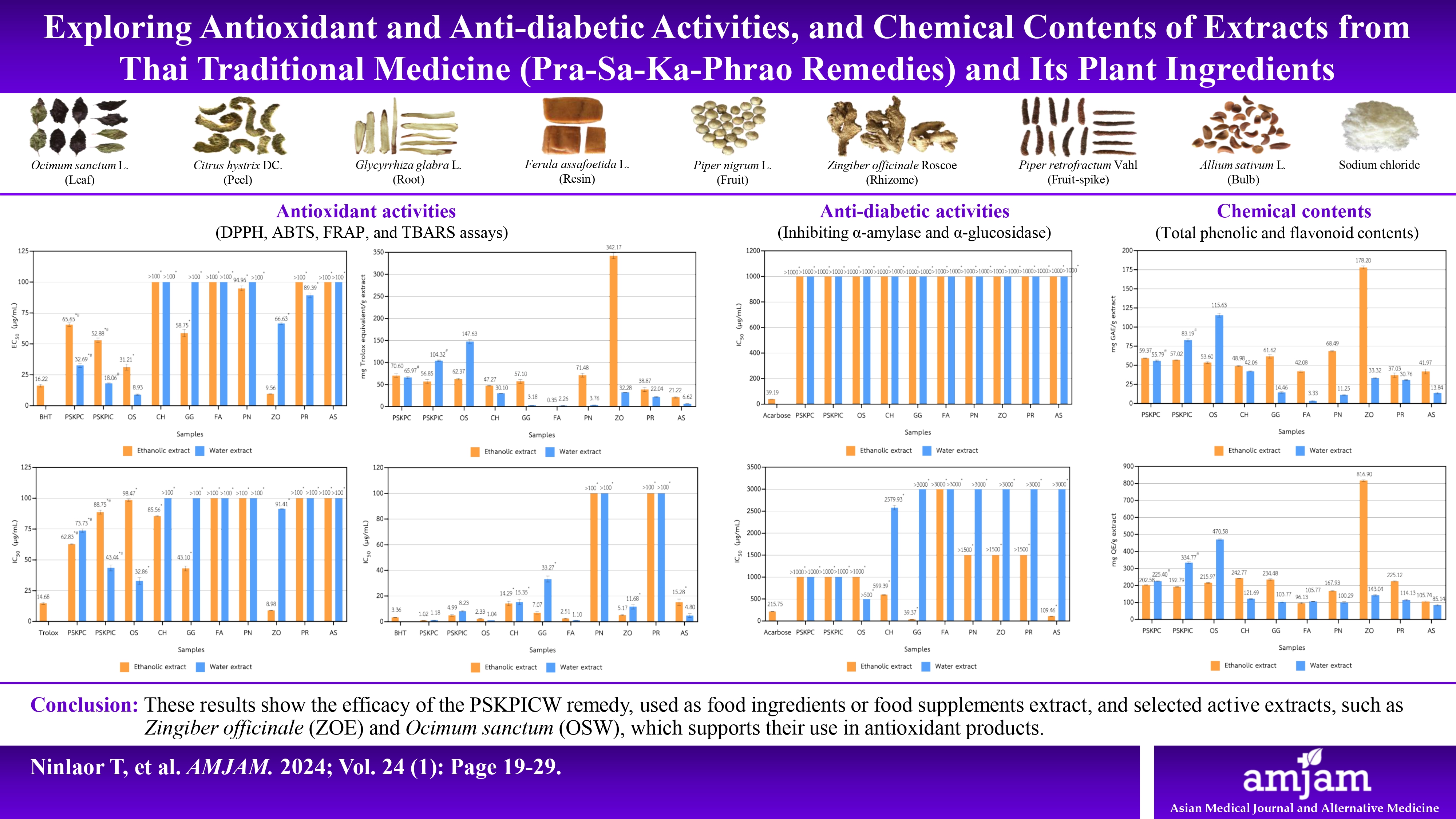
Methods: Extraction was performed by maceration in 95% ethanol and decoction. The antioxidant activity was investigated using a DPPH, ABTS, FRAP, and TBARS assays, while the anti-diabetic (α-amylase, α-glucosidase inhibitory activities) were also evaluated, along with the determination of total phenolic (TPC) and total flavonoid (TFC) contents.
Results: The ethanolic extract of Zingiber officinale (ZOE) and water extract of Ocimum sanctum (OSW) exhibited the highest antioxidant activity, TPC, and TFC contents. The antioxidant results revealed that the PSKPIC water extract (PSKPICW) showed greater potency than PSKPC water extract in all assays. Additionally, the PSKPICW demonstrated higher TPC and TFC levels compared to the PSKPC remedy. Glycyrrhiza glabra (GGE) presented the strongest α-glucosidase inhibitory activity. However, all remedy extracts did not significantly affect anti-diabetic activity.
Conclusions: These results show the efficacy of the PSKPICW remedy, used as food ingredients or food supplements extract, and selected active extracts, such as ZOE and OSW, which supports their use in antioxidant products.
Downloads
References
Seyedsadjadi N, Grant R. The Potential Benefit of Monitoring Oxidative Stress and Inflammation in the Prevention of Non-Communicable Diseases (NCDs). Antioxidants. 2020;10(1):15.
Peña-Oyarzun D, Bravo-Sagua R, Diaz-Vega A, et al. Autophagy and oxidative stress in non-communicable diseases: A matter of the inflammatory state? Free Radic Biol Med. 2018;124:61-78.
Burgos-Morón, Abad-Jiménez, Marañón, et al. Relationship Between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J Clin Med. 2019;8(9):1385.
Angelova PR, Esteras N, Abramov AY. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med Res Rev. 2020;41(2):770-784.
The Thai Food and Drug Administration. A list of the plants allowed for food supplement 2017. Bangkok: The Thai Food and Drug Administration; 2017 Aug 7:1-53. https://food.fda.moph.go.th/media.php?id=509562086569943040&name=PlantName. Published 2017. Accessed February 18, 2019.
The Thai Food and Drug Administration. A list of the plants allowed for food supplement (No.2). Bangkok: The Thai Food and Drug Administration; 2018 Aug 22:1-10. https://food.fda.moph.go.th/media.php?id=509583079023714304&name=PlantName_2. Published 2018. Accessed February 18, 2019.
Suanarunsawat T, Anantasomboon G, Piewbang C. Anti-diabetic and anti-oxidative activity of fixed oil extracted from Ocimum sanctum L. leaves in diabetic rats. Exp Ther Med. 2016;11(3):832-840.
Warsito W, Noorhamdani N, Sukardi S, Suratmo S. Assessment of antioxidant activity of citronellal extract and fractions of essential oils of Citrus hystrix DC. Trop J Pharm Res. 2018;17(6):1119.
Sen S, Roy M, Chakraborti AS. Ameliorative effects of glycyrrhizin on streptozotocininduced diabetes in rats. J Pharm Pharmacol. 2011;63(2):287-296.
Yousfi F, Abrigach F, Petrovic JD, Sokovic M, Ramdani M. Phytochemical screening and evaluation of the antioxidant and antibacterial potential of Zingiber officinale extracts. S Afr J Bot. 2021;142:433-440.
Ghafoor K, Al Juhaimi F, Özcan MM, Uslu N, Babiker EE, Mohamed Ahmed IA. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. LWT. 2020;126:109354.
Jadid N, Hidayati D, Hartanti SR, Arraniry BA, Rachman RY, Wikanta W. Antioxidant activities of different solvent extracts of Piper retrofractum Vahl. using DPPH assay. AIP Conf Proc. 2017;1854(1):020019.
Yamasaki K, Hashimoto A, Kokusenya Y, Miyamoto T, Sato T. Electrochemical Method for Estimating the Antioxidative Effects of Methanol Extracts of Crude Drugs. Chem Pharm Bull. 1994;42(8):1663-1665.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9-10):1231-1237.
Benzie IFF, Szeto YT. Total Antioxidant Capacity of Teas by the Ferric Reducing/Antioxidant Power Assay. J Agric Food Chem. 1999;47(2):633-636.
Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000;69(2):167-174.
Yuan G, Li W, Pan Y, Wang C, Chen H. Shrimp shell wastes: Optimization of peptide hydrolysis and peptide inhibition of α-amylase. Food Biosci. 2018;25:52-60.
Wongnawa M, Tohkayomatee R, Bumrungwong N, Wongnawa S. Alpha-glucosidasae inhibitory effect and inorganic constituents of Phyllanthus amarus Schum. & Thonn. ash. Songklanakarin J Sci Technol. 2014;36(5):541-546.
Miliauskas G, Venskutonis PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85(2):231-237.
Zhu H, Wang Y, Liu Y, Xia Y, Tang T. Analysis of Flavonoids in Portulaca oleracea L. by UV-Vis Spectrophotometry with Comparative Study on Different Extraction Technologies. Food Anal Methods. 2009;3(2):90-97.
Catalán V, Frühbeck G, Gómez-Ambrosi J. Inflammatory and oxidative stress markers in skeletal muscle of obese subjects. Obesity. 2018:163-189.
Chaudhary A, Sharma S, Mittal A, Gupta S, Dua A. Phytochemical and antioxidant profiling of Ocimum sanctum. J Food Sci Technol. 2020;57(10):3852-3863.
Ali AMA, El-Nour MEM, Yagi SM. Total phenolic and flavonoid contents and anti-oxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J Genet Eng Biotechnol. 2018;16(2):677-682.
Loizzo MR, Formoso P, Leporini M, Sicari V, Falco T, Tundis R. Influence of Organic and Conventional Agricultural Practices on Chemical Profile, In Vitro Antioxidant and Anti-Obesity Properties of Zingiber officinale Roscoe. The 1st International e-Conference on Antioxidants in Health and Disease. 2020;2(1):3.
Wangcharoen W, Morasuk W. Antioxidant capacity and phenolic content of holy basil. Songklanakarin J Sci Technol. 2007;29(5):1407-1415.
Diaz-Flores J, Ybañez-Julca RO, Asunción-Alvarez D, Quispe-Díaz IM, Asmat-Marrufo P. Capacidad antioxidante in vitro del liofilizado de la pulpa y cáscara del rizoma de Zingiber officinale Roscoe (jengibre). Rev Peru Med Integr. 2019;4(4):121-126.
Gholam HA, Falah H, Sharififar F, Mirtaj AS. The inhibitory effect of some Iranian plants extracts on the alpha glucosidase. Iran J Basic Med Sci. 2008;11(1):1-9.
Molan AL, Saleh Mahdy A. Total Phenolics, Antioxidant Activity and Anti-Diabetic Capacities of Selected Iraqi Medicinal Plants. Am J Life Sci Res. 2016;4(2):47-59.
Belemkar S, Dhameliya K, Pata MK. Comparative study of garlic species (Allium sativum and Allium porrum) on glucose uptake in diabetic rats. J Taibah Univ Med Sci. 2013;8(2):80-85.
Thomson M, Al-Qattan KK, JS D, Ali M. Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;16(1):17.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Asian Medical Journal and Alternative Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.


