Quality Control of Raw Plant Materials and Stability Testing under Accelerated Storage Conditions of Kheaw-Hom Remedy Extract
Keywords:
Quality control, Stability testing, Accelerated storage conditions, Kheaw-HomAbstract
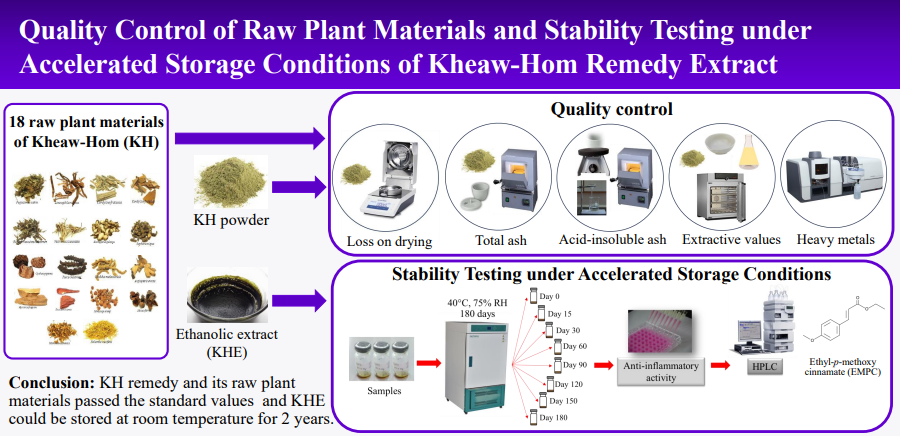
Introduction: Kheaw-Hom remedy (KH), a Thai traditional antipyretic medicine, is included in the National List of Essential Medicine. Currently, there is no scientific report on its standard requirements for quality control and stability testing.
Objectives: To determine the quality control and stability testing of KH on anti-inflammatory activity and its bioactive marker
Methods: Quality control methods (loss on drying, total ash, acid-insoluble ash, extractive values, and heavy metals) of KH and raw plant materials were performed according to Thai Herbal Pharmacopoeia (THP). The stability testing of the ethanolic extract of KH (KHE) was stored under 40 ± 2 ºC and 75 ± 5% relative humidity for 180 days. Anti-inflammatory activity on LPS-induced nitric oxide (NO) production in RAW264.7 macrophage cells were evaluated. Ethyl p-methoxycinnamate (EPMC), a bioactive marker, was analyzed using high performance liquid chromatography (HPLC).
Results: KH showed loss on drying, total ash, acid-insoluble ash, ethanol soluble extractive and water-soluble extractive values of 8.66 ± 0.47%, 6.17 ± 0.06%, 1.14 ± 0.07%, 10.62 ± 0.12%, and 13.78 ± 0.54%, respectively. Eighteen plant materials met the requirements of THP. The anti-inflammatory activity on nitric oxide inhibition and EPMC content of KHE on day 180 exhibited no significant difference when compared with day 0.
Conclusions: This study is the first report on quality control and stability testing of KH. All KH and its plant components conformed to the standard requirements of THP. KHE could be stored at room temperature for two years.
Downloads
References
Notification of the National Drug System Development Committee Re: National List of Essential Medicines. Ministry of Public Health. http://dmsic.moph.go.th/index/download/852. Published 2021. Accessed June 12, 2023.
Sanguansermsri D, Lamlertthon S, Pongcharoen S, Preechanukool K, Chumpol S, Promkhatkaew D. Anti-varicella zoster virus of Ya-keaw remedies. Thailand Science Research and Innovation. http://elibrary.trf.or.th/project_content.asp?PJID=MRG4680174. Published June 30, 2005. Accessed June 12, 2023.
Chaihad P, Pholor N, Khamseetha P, Kongmuang S, Pongpirul K, Phadungkit M. Efficacy and safety of Ya-Khiao-Hom powder for the treatment of aphthous ulcer. J Thai Trad Alt Med. 2021; 19(2):344-353.
Khamsetha P, Chaihad P, Kongmuang S, et al. Oral and topical application of Kheaw Hom powder on oral ulcer: efficacy and patient preference (KHOU). Gut. 2019;68(1):A77-A77.
Kongkeaw P, Seba N. Comparative study of tepid sponging using Ya Khiao-Hom and traditional method for reducing body temperatures on pediatric patients with pneumonia and high fever: A Retrospective Case-Cohort Study. J Thai Trad Alt Med. 2023;21(1):18-24.
Sukkasem K. Biological activities of Thai traditional remedy called Kheaw-Hom and its plant ingredients. [Master’s thesis]. Faculty of Medicine: Thammasat University; 2015.
Ouncharoen K, Itharat A, Chaiyawatthanananthn P. In vitro free radical scavenging and cell-based antioxidant activities of Kheaw-Hom remedy extracts and its plant ingredients. J Med Assoc Thai. 2017;100(6):241-249.
Sukkasem K, Panthong S, Itharat A. Antimicrobial activities of Thai traditional remedy “Kheaw-Hom” and its plant ingredients for skin infection treatment in chickenpox. J Med Assoc Thai. 2016;99(4):116-123.
Chaniad P, Techarang T, Phuwajaroanpong A, et al. Exploring potential antimalarial candidate from medicinal plants of Kheaw Hom remedy. Trop Med Infect Dis. 2022;7(11):368.
Thai Herbal Preparation Pharmacopoeia. Department of Thai Traditional and Alternative Medicine, Ministry of Public Health. https://thpp.dtam.moph.go.th/. Published 2022. Accessed June 12, 2023.
Thai Herbal Pharmacopoeia. Department of Medical Science, Ministry of Public Health. https://bdn.go.th/thp/home. Published 2021. Accessed June 12, 2023.
Department of Thai Traditional and Alternative Medicine, Ministry of Public Health. Quality control of Thai Herbal Preparation. 2nd ed. Bangkok: Chulalongkorn University Press; 2021.
European Medicine Agency. ICH topic Q1A (R2) stability testing of new drug substances and products, Note for guidance on stability testing: stability testing of new drug substances and products (CPMP/ICH/2736/99). London, England: EMEA; 2006.
World Health Organization. Quality control methods for herbal materials. Geneva: World Health Organization Press; 2011.
Jansom C, Jansom V. Development and validation of analytical methods for arsenic and mercury determination by atomic absorption spectrophotometry. Tham Med J. 2019;19(1):25-35.
Duangteraprecha S, Jamtaweekul J, Sukphan P, Inthongkaew P. Method development and validation for determination of arsenic, lead and cadmium in herbal medicines by graphite furnace atomic absorption spectrophotometry. Bull Dept Med Sci. 2015;1:20-33.
Makchuchit S, Rattarom R, Itharat A. The anti-allergic and anti-inflammatory effects of Benjakul extract (a Thai traditional medicine), its constituent plants and its some pure constituents using in vitro experiments. Biomed Pharmacother. 2017;89:1018-1026.
Chokevivat V. Quality of crude drug. J Thai Trad Alt Med. 2004;2:84-91.
Issaravanich S, Palanuvej C, Tunsaringkarn T, et al. Pharmacognostic specification of Vetiveria zizanioides roots in Thailand. J Health Res. 2008;22(1):9-14.
Sukkasem K, Itharat A, Thisayakorn K, et al. Exploring in vitro and in vivo anti-inflammatory activities of the Thai traditional remedy Kheaw-Hom and its bioactive compound, ethyl p-methoxycinnamate, and ethnopharmacological analysis. J Ethnopharmacol. 2024;319:117131.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Asian Medical Journal and Alternative Medicine

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.


